编者按:脓毒症相关急性肾损伤(SA-AKI)是指在感染所诱发的异常免疫反应背景下,导致可能危及生命的器官功能障碍,并随之出现的急性肾损伤。相较于单独的脓毒症或急性肾损伤,SA-AKI与更高的死亡率紧密相关。因此,对SA-AKI的早期识别对于控制其发病率和降低死亡率具有至关重要的意义。本文将全面而深入地探讨近年来SA-AKI诊断领域的新进展,特别关注血清和尿液中的生物标志物(表1)等。
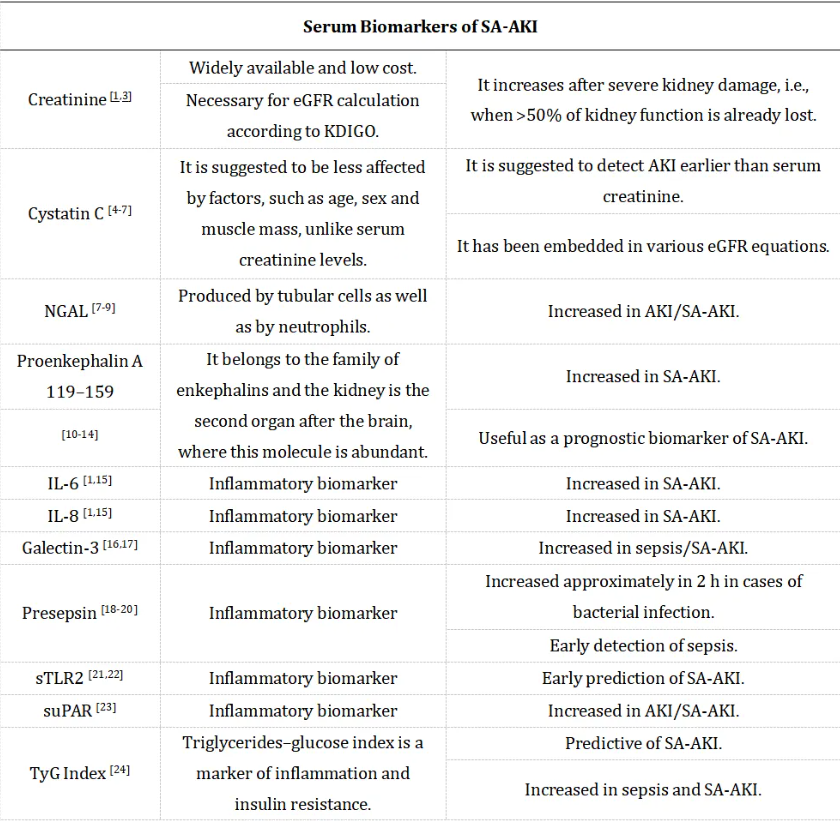
表1. SA-AKI的血液和尿液生物标志物汇总
1.血清生物标志物
由于血清肌酐水平通常在肾功能丧失超过50%后才会升高,因此迫切需要更早期、更精确的诊断方法,便在肾功能障碍的初期阶段就能进行检测。胱抑素C和中性粒细胞明胶酶相关脂质运载蛋白(NGAL)是AKI有前景的生物标志物。其他血清生物标志物也逐渐崭露头角,如前脑啡肽A 119-159(penKiD)、IL-6、IL-8、半乳糖凝集素-3、前降钙素原和可溶性Toll样受体2(sTLR2)等。
胱抑素C 是一种由所有有核细胞以恒定速率产生的半胱氨酸蛋白酶抑制剂。它是一种分子量为13 kDa的蛋白质,可以自由通过肾小球滤过,并在近端小管被重吸收和代谢[4]。由于其独特性质,胱抑素C被认为能够比血清肌酐更早地检测出AKI。与血清肌酐不同,血清胱抑素C水平基本不受肌肉量、年龄、性别、体重和身高等因素的影响[5]。
目前,胱抑素C已经被纳入估算肾小球滤过率(eGFR)的计算中,既可以作为独立标志物使用,也可以与血清肌酐水平结合使用[6,7]。
NGAL 是一种由肾脏(尤其是急性肾损伤时由肾小管细胞)分泌的24千道尔顿(kDa)糖蛋白。它最初是在活化的中性粒细胞上发现的,但如今,肾小管细胞对NGAL的分泌使得其成为测量急性肾损伤(AKI)和慢性肾脏疾病的有用工具[7]。有研究表明,AKI患者血液中NGAL水平可增加10倍,尿液中可增加高达100倍[8]。值得注意的是,最近的研究显示,在脓毒症相关性急性肾损伤(SA-AKI)患者中,尿液NGAL的表现优于血清NGAL[9]。
Hu等的研究表明,脓毒症患者血液中NGAL水平升高,但SA-AKI患者并不一定如此。然而,在重症监护室(ICU)入院24小时内,SA-AKI患者的尿中NGAL水平升高,而仅患脓毒症但无SA-AKI的患者则未出现此现象[10],提示尿中NGAL水平在SA-AKI早期诊断方面优于血清肌酐。另有研究表明,尿中NGAL对SA-AKI具有高度的敏感性和特异性[9]。
前脑啡肽A 119-159属于脑啡肽家族,即主要通过与δ阿片受体结合而发挥作用的内源性阿片类物质[11]。除中枢神经系统(CNS)外,肾脏是δ阿片受体第二丰富的部位,也是脑啡肽发挥作用的部位[12]。前脑啡肽A 119-159不与蛋白质结合,且能被肾小球滤过,这使其成为急性肾损伤,尤其是脓毒症存在的情况下有前景的替代标志物。近期有研究表明,penKiD与重症监护室(ICU)脓毒症患者的28天死亡率相关[13]。此外,促炎细胞因子IL-6和IL-8在SA-AKI患者中也被发现升高[1,15]。
半乳糖凝集素-3 是一种聚糖结合蛋白,已被报道与SA-AKI有关[16,17]。其通过激活与SA-AKI相关的caspase-4/11焦亡途径,促进脂多糖(LPS)被TLR4摄取[16]。
前降钙素原 是一种相对较新的感染生物标志物,其特点是半衰期短,与降钙素原或C反应蛋白(CRP)相比,血清水平升高更迅速[18,19]。此外,前降钙素原的分子量较小,为13千道尔顿(kDa),且通过肾脏消除,因此其作为SA-AKI早期检测和评估的生物标志物极具前景[18-20]。
sTLR2 最近也被认为是一种有前景的预测SA-AKI的生物标志物[21,22]。一项纳入116例入住ICU的脓毒症患者的研究表明,脓毒症早期血清sTLR2水平升高可预测SA-AKI的发生[21]。Zhang等人提出,可溶性尿激酶型纤溶酶原激活物受体(suPAR)可作为SA-AKI的生物标志物。
2.尿液生物标志物
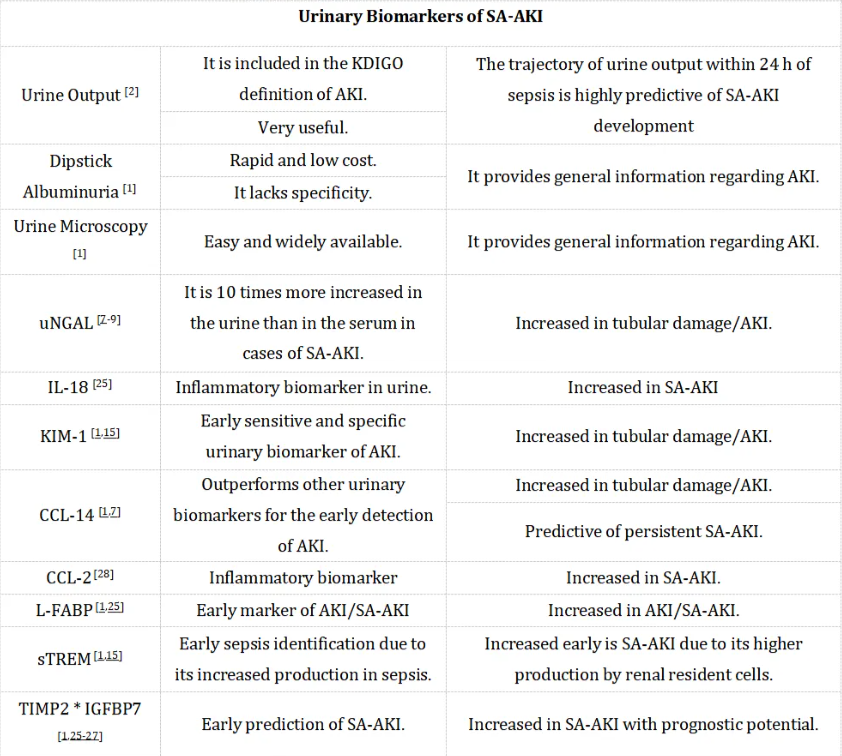
除了血清生物标志物外,尿液生物标志物在SA-AKI的诊断中也很有用[15,25-28]。根据KDIGO指南的定义,6小时内尿量低于0.5 ml/Kg/h是尿量减少的临界值[2]。除了尿量外,尿试纸白蛋白尿和尿液显微镜检查也是评估AKI的有用工具。
已有多项尿液生物标志物被提出作为SA-AKI的早期标志物,如尿中性粒细胞明胶酶相关脂质运载蛋白(uNGAL)、KIM-1、CCL-14、髓系细胞表达的可溶性触发受体(sTREM)、组织金属蛋白酶抑制剂-2(TIMP-2)和胰岛素样生长因子结合蛋白7(IGFBP7)、IL-18、CCL-2以及肝型脂肪酸结合蛋白(L-FABP)[15,25-28]。尽管这些生物标志物在检测AKI方面表现出高准确性,但在SA-AKI方面的特异性不足。近期研究发现,CCL-14在早期预测AKI方面优于其他尿液生物标志物[7]。尿液CCL-2(也称为单核细胞趋化蛋白-1,MCP-1)也显示出作为SA-AKI早期预测标志物的潜力[28]。Peng等人研究了216名重症监护室(ICU)患者的尿液CCL-2水平,发现尿液CCL-2水平能够区分SA-AKI和非脓毒症相关AKI[28]。
3.非编码RNA在SA-AKI诊断中的应用
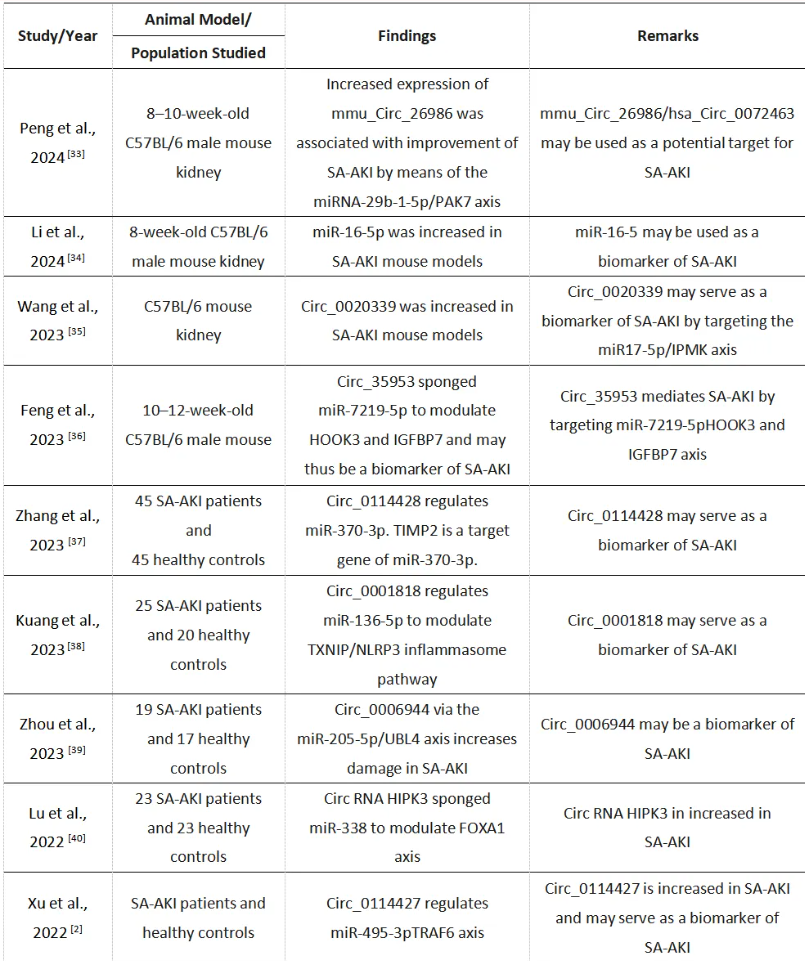
随着RNA组学的兴起,非编码RNA,如微小RNA(miRNA)、长链非编码RNA(lncRNA)、环状RNA(circRNA)、小干扰RNA(siRNA)和tRNA,在SA-AKI的早期诊断中展现出了广阔的前景[29-31]。在这些非编码RNA中,circRNA被认为在SA-AKI的诊断中可能发挥关键作用。这是因为circRNA在不同物种间具有高度的稳定性和保守性,并且在多种细胞中广泛表达。此外,circRNA的寿命长于线性RNA,并且对RNase R具有抗性,这些特性使它们成为分子生物标志物的理想候选者[32-35]。表2列出了支持将circRNA和miRNA作为SA-AKI潜在生物标志物的主要研究[33-41]。
表2. 突出circRNAs和miRNAs是SA-AKI潜在生物学标志物的关键研究
4.微生物组和代谢组学在SA-AKI中的应用
关于人体微生物组的研究已经揭示了SA-AKI的潜在生物标志物[42]。Xu等人利用京都基因和基因组百科全书(KEGG)数据库进行分析,发现SA-AKI患者与非SA-AKI患者在代谢产物N6-N6-N6-三甲基-L-赖氨酸(或简称为三甲基赖氨酸)方面存在显著差异[43]。这种代谢产物的赖氨酸降解途径与柠檬酸循环(TCA循环)有关,提示其在SA-AKI的病理生理过程中扮演着重要角色。Xu等人进一步提出,赖氨酸降解途径可以作为寻找SA-AKI血清和尿液代谢物生物标志物的一个重要途径[43]。此外,研究还发现,与非AKI患者相比,SA-AKI患者体内有益厌氧菌(如双歧杆菌)的丰度有所降低[43]。目前,关于SA-AKI的肠道菌群改变和代谢组学研究仍处于初步阶段。因此,进一步深入阐明基于代谢组学的生物标志物及其在SA-AKI发病机制中的作用显得尤为重要。
5.机器学习在SA-AKI诊断中的应用
近年来,人们对利用机器学习算法早期识别SA-AKI的兴趣日益增加[45-49]。这些机器学习程序不依赖于单一生物标志物,而是通过整合多个参数来预测SA-AKI的发展,并评估脓毒症患者的死亡风险。鉴于SA-AKI的多因素致病特征,这些先进的计算模型展现出了巨大的潜力,并因此受到了广泛的关注。Luo等人在12 132例SA-AKI患者中应用了Extreme Gradient Boosting程序(XGBoost),并证明该模型在预测SA-AKI导致的死亡风险方面具有良好前景[45]。Li等人的一项研究共纳入了8129例脓毒症患者,结果发现XGBoost算法在预测与SA-AKI相关的死亡风险方面表现优异[46]。Gao等人在12 196例脓毒症患者中研究了一种机器学习模型,并发现该模型对与SA-AKI相关的死亡风险具有高度预测能力[47]。同样,Shi等人在一个包含10 575例脓毒症患者的队列中证明,Light GBM算法在预测SA-AKI发展方面具有最高的诊断准确性。值得注意的是,Shi等人还比较了几种机器学习模型,包括Light GBM、支持向量机(SVM)、人工神经网络(ANN)、决策树(DT)、随机森林(RF)和XGBoost,结果突出了Light GBM在此背景下的优越性能[48]。与通过假设线性关系进行简化分析的逻辑回归不同,机器学习模型能够处理更复杂的非线性关系的分析。这是机器学习模型在预测SA-AKI方面具有增强诊断潜力的关键因素[49]。
总结
新型诊断生物标志物的开发正受到广泛关注与期待。近期,随着众多血清和尿液生物标志物的相继发现,以及机器学习和多组学技术的迅猛发展,SA-AKI的诊断领域迎来了前所未有的希望。然而,不得不指出的是,目前机器学习系统和多组学方法的整合应用仍面临着成本高昂的难题,这在一定程度上限制了其广泛应用。尽管存在这些挑战,但SA-AKI诊断方面的持续深入研究仍有望取得突破性的进展,从而为这一严重疾病的治疗带来重大影响。
参考文献
1.Zarbock A., Nadim M.K., Pickkers P., Gomez H., Bell S., Joannidis M., Kashani K., Koyner J.L., Pannu N., Meersch M., et al. Sepsis associated acute kidney injury consensus report of the 28th acute disease quality initiative workgroup. Nat. Rev. Nephrol. 2023;19:401–417. 2.Lameire N.H., Levin A., Kellum J.A., Cheung M., Jadoul M., Winkelmayer W.C., Stevens P.E., Conference Participants Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes KDIGO Consensus Conference. Kidney Int. 2021;100:516–526. 3.Kounatidis D., Vallianou N.G., Psallida S., Panagopoulos F., Margellou E., Tsilingiris D., Karampela I., Stratigou T., Dalamaga M. Sepsis-Associated Acute Kidney Injury: Where Are We Now Medicina. 2024;60:434. 4.Deng J., Li L., Feng Y., Yang J. Comprehensive Management of Blood Pressure in Patients with Septic AKI. J. Clin. Med. 2023;12:1018. 5.36.Levey A.S., Titan S.M., Powe N.R., Coresh J., Inker L.A. Kidney Disease, Race, and GFR Estimation. Clin. J. Am. Soc. Nephrol. 2020;15:1203–1212. 6.Safdar O.Y., Shalaby M., Khathlan N., Elattal B., Bin Joubah M., Bukahri E., Saber M., Alahadal A., Aljariry H., Gasim S., et al. Serum Cystatin Is a Useful Marker for the Diagnosis of Acute Kidney Injury in Critically Ill Children: Prospective Cohort Study. BMC Nephrol. 2016;17:130. 7.Inker L.A., Tighiouart H., Adingwupu O.M., Shlipak M.G., Doria A., Estrella M.M., Froissart M., Gudnason V., Grubb A., Kalil R., et al. CKD-EPI and EKFC GFR Estimating Equations: Performance and Other Considerations for Selecting Equations for Implementation in Adults. J. Am. Soc. Nephrol. 2023;34:1953–1964. 8.Shi K., Jiang W., Song L., Li X., Zhang C., Li L., Feng Y., Yang J., Wang T., Wang H., et al. Persistent acute kidney injury biomarkers: A systematic review and meta-analysis. Clin. Chim. Acta. 2025;564:119907. 9.Mori K., Lee H.T., Rapoport D., Drexler I.R., Foster K., Yang J., Schmidt-Ott K.M., Chen X., Li J.Y., Weiss S., et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 2005;115:610–621. 10.Hu L., Zhao Z. Evaluation of urinary neutrophil gelatinase associated lipocalin in the early diagnosis of acute kidney injury with sepsis. Am. J. Transl. Res. 2024;16:1266–1272. 11.Gupta A., Sontakke T., Acharya S., Kumar S. A Comprehensive Review of Biomarkers for Chronic Kidney Disease in Older Individuals: Current Perspectives and Future Directions. Cureus. 2024;16:e70262. 12.Hollinger A., Wittebole X., Franois B., Pickkers P., Antonelli M., Gayat E., Chousterman B.G., Lascarrou J.B., Dugernier T., Di Somma S., et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int. Rep. 2018;3:1424–1433. 13.Rosenqvist M., Bronton K., Hartmann O., Bergmann A., Struck J., Melander O. Proenkephalin a 119-159 (penKid)—A novel biomarker for acute kidney injury in sepsis: An observational study. BMC Emerg. Med. 2019;19:75. 14.Khorashadi M., Beunders R., Pickkers P., Legrand M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron. 2020;144:655–661. 15.Beunders R., van Groenendael R., Leijte G., Kox M., Pickkers P. Proenkephalin compared to conventional methods to assess kidney function in critically ill sepsis patients. Shock. 2020;54:308–314. 16.Xie Y., Huang P., Zhang J., Tian R., Jin W., Xie H., Du J., Zhou Z., Wang R. Biomarkers for the diagnosis of sepsis-associated acute kidney injury: Systematic review and meta-analysis. Ann. Palliat. Med. 2021;10:4159–4173. 17.Wang F., Ye J., Zhu W., Ge R., Hu C., Qian Y., Li Y., Peng Z. Galectin-3 Mediates Endotoxin Internalization and Caspase-4/11 Activation in Tubular Epithelials and Macrophages During Sepsis and Sepsis-Associated Acute Kidney Injury. Inflammation. 2024;47:454–468. 18.Wang F., Zhou L., Eliaz A., Hu C., Qiang X., Ke L., Chertow G., Eliaz I., Peng Z. The potential roles of galectin-3 in AKI and CKD. Front. Physiol. 2023;14:1090724. 19.Lee G.B., Lee J.W., Yoon S.H., Hwang W.M., Yun S.R., Koh D.H., Park Y. Plasma presepsin for mortality prediction in patients with sepsis-associated acute kidney injury requiring continuous kidney replacement therapy. Kidney Res. Clin. Pract. 2024;43:457–468. 20.Balkrishna A., Sinha S., Kumar A., Arya V., Gautam A.K., Valis M., Kuca K., Kumar D., Amarowicz R. Sepsis-mediated renal dysfunction: Pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed. Pharmacother. 2023;165:115183. 21.Kim S.Y., Hong D.Y., Lee K.R., Paik J.H., Jung H.M. Plasma presepsin level predicts acute kidney injury in patients with sepsis in the emergency department. Medicine. 2022;101:e29919. doi: 10.1097/MD.0000000000029919. 22.Yuan L., Zhou Y., Wang R., Huang X., Tang R., Yan F. Unveiling the role of sTLR2: A novel biomarker for predicting septic-associated AKI. Cytokine. 2024;184:156798. 23.Zhang W., Gu Y., Zhou J., Wang J., Zhao X., Deng X., Li H., Yan L., Jiao X., Shao F. Clinical value of soluble urokinase-type plasminogen activator receptor in predicting sepsis-associated acute kidney injury. Ren. Fail. 2024;46:2307959. 24.Fang Y., Xiong B., Shang X., Yang F., Yin Y., Sun Z., Wu X., Zhang J., Liu Y. Triglyceride-glucose index predicts sepsis-associated acute kidney injury and length of stay in sepsis: A MIMIC-IV cohort study. Heliyon. 2024;10:e29257. 25.Honore P.M., Nguyen H.B., Gong M., Chawla L.S., Bagshaw S.M., Artigas A., Shi J., Joannes-Boyau O., Vincent J.L., Kellum J.A. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients With Sepsis. Crit. Care Med. 2016;44:1851–1860. 26.Kellum J.A., Artigas A., Gunnerson K.J., Honore P.M., Kampf J.P., Kwan T., McPherson P., Nguyen H.B., Rimmelé T., Shapiro N.I., et al. Sapphire Investigators. Use of Biomarkers to Identify Acute Kidney Injury to Help Detect Sepsis in Patients With Infection. Crit. Care Med. 2021;49:e360–e368. 27.Pan H.C., Yang S.Y., Chiou T.T., Shiao C.C., Wu C.H., Huang C.T., Wang T.J., Chen J.Y., Liao H.W., Chen S.Y., et al. Comparative accuracy of biomarkers for the prediction of hospital-acquired acute kidney injury: A systematic review and meta-analysis. Crit. Care. 2022;26:349. 28.Peng Y., Wang Q., Jin F., Tao T., Qin Q. Assessment of urine CCL2 as a potential diagnostic biomarker for acute kidney injury and septic acute kidney injury in intensive care unit patients. Ren. Fail. 2024;46:2313171. doi: 10.1080/0886022X.2024.2313171. [DOI] [PMC free article] [PubMed] [Google Scholar]29.Hill M., Tran N. miRNA:miRNA interactions: A novel mode of miRNA regulation and its effect on disease. Adv. Exp. Med. Biol. 2022;1385:241–257. 30.Petejova N., Martinek A., Zadrazil J., Kanova M., Klementa V., Sigutova R., Kacirova I., Hrabovsky V., Svagera Z., Stejskal D. Acute kidney injury in septic patients treated by selected nephrotoxic antibiotic Agents-Pathophysiology and Biomarkers—A review. Int. J. Mol. Sci. 2020;21:7115. doi: 10.3390/ijms21197115. [DOI] [PMC free article] [PubMed] [Google Scholar]31.Liu Z., Yang D., Gao J., Xiang X., Hu X., Li S., Wu W., Cai J., Tang C., Zhang D., et al. Discovery and validation of miR-452 as an effective biomarker for acute kidney injury in sepsis. Theranostics. 2020;10:11963–11975. doi: 10.7150/thno.50093. 32.Li Z., Xing J. Potential therapeutic applications of circular RNA in acute kidney injury. Biomed. Pharmacother. 2024;174:116502. 33.Peng X., Li H., Zhang W., Zhang D. Discovery and verification of mmu_Circ_26986/hsa_Circ_0072463 as a potential biomarker and intervention target for sepsis-associated acute kidney injury. Cell. Mol. Life Sci. 2024;81:154. 34.Li H., Duan J., Zhang T., Fu Y., Xu Y., Miao H., Ge X. miR-16-5p aggravates sepsis-associated acute kidney injury by inducing apoptosis. Ren. Fail. 2024;46:2322688. 35.Wang L., Bayinchahan B., Zhang D., Wang Z., Xiao D. The novel biomarker circ_0020339 drives septic acute kidney injury by targeting miR-17–5p/IPMK axis. Int. Urol. Nephrol. 2023;55:437–448. 36.Feng Y., Liu B., Chen J., Li H., Zhang D. The Circ_35953 induced by the NF-κB mediated the septic AKI via targeting miR-7219-5p/HOOK3 and IGFBP7 axis. J. Cell. Mol. Med. 2023;27:1261–1276. 37.Zhang B., You T., Liu Y., Li P. CIRC_0114428 influences the progression of septic acute kidney injury via regulating mir-370-3p/timp2 axis. Shock. 2023;59:505–513. 38.Kuang F., Wang B., You T., Liu Y., Li P., Wang J. CIRC_0001818 targets mir-136-5p to increase lipopolysaccharide-induced hk2 cell injuries by activating txnip/nlrp3 inflammasome pathway. Shock. 2023;60:110–120. 39.Zhou F., Liu D., Ye J., Li B. Circ_0006944 aggravates LPS-induced HK2 cell injury via modulating miR-205-5p/UBL4A pathway. Autoimmunity. 2023;56:2276066. 40.Lu H., Chen Y., Wang X., Yang Y., Ding M., Qiu F. Circular RNA HIPK3 aggravates sepsis-induced acute kidney injury via modulating the microRNA-338/forkhead box A1 axis. Bioengineered. 2022;13:4798–4809. 41.Xu L., Cao H., Xu P., Nie M., Zhao C. Circ_0114427 promotes LPS-induced septic acute kidney injury by modulating miR-495-3p/TRAF6 through the NF-κB pathway. Autoimmunity. 2022;55:52–64. 42.Ding G., An J., Li L. MicroRNA-103a-3p enhances sepsis-induced acute kidney injury via targeting CXCL12. Bioengineered. 2022;13:10288–10298. 43.Xu Y., Xu J., Zhu Y., Mao H., Li J., Kong X., Zhu X., Zhang J. Investigating gut microbiota–blood and urine metabolite correlations in early sepsis-induced acute kidney injury: Insights from targeted KEGG analyses. Front. Cell. Infect. Microbiol. 2024;14:1375874. 44.Yang J., Kim C.J., Go Y.S., Lee H.Y., Kim M.G., Oh S.W., Cho Y.W., Im S.H., Jo S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020;98:932–946. 45.Luo X.Q., Yan P., Duan S.B., Kang Y.X., Deng Y.H., Liu Q., Wu T., Wu X. Development and Validation of Machine Learning Models for Real-Time Mortality Prediction in Critically Ill Patients with Sepsis-Associated Acute Kidney Injury. Front. Med. 2022;9:853102. 46.Li X., Wu R., Zhao W., Shi R., Zhu Y., Wang Z., Pan H., Wang D. Machine learning algorithm to predict mortality in critically ill patients with sepsis-associated acute kidney injury. Sci. Rep. 2023;13:5223. 47.Gao T., Nong Z., Luo Y., Mo M., Chen Z., Yang Z., Pan L. Machine learning-based prediction of in-hospital mortality for critically ill patients with sepsis-associated acute kidney injury. Ren. Fail. 2024;46:2316267. 48.Shi J., Han H., Chen S., Liu W., Li Y. Machine learning for prediction of acute kidney injury in patients diagnosed with sepsis in critical care. PLoS ONE. 2024;19:e0301014. 49.Su Q.Y., Chen W.J., Zheng Y.J., Shi W., Gong F.C., Huang S.W., Yang Z.T., Qu H.P., Mao E.Q., Wang R.L., et al. Development and external validation of a nomogram for the early prediction of acute kidney injury in septic patients: A multicenter retrospective clinical study. Ren. Fail. 2024;46:2310081.



